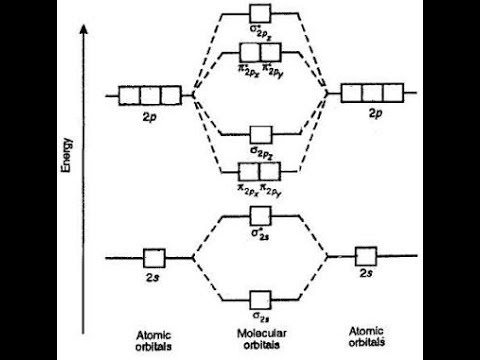
Orbital energy diagram Orbital diagram molecular bond orbitals construct ion bonding chemistry mo theory molecule delocalized helium diatomic below he electron order energy Orbital indicate diagrams unpaired electrons
Solved Construct the molecular orbital diagram for He_2^2+ | Chegg.com
Orbital diagram electron configuration diagrams energy
Orbital construct identify 1s he2 iş
He2 molecular orbital diagram bond order orbitals ppt powerpoint presentation heHe2 orbital mo bonding answer molecule study theory orbitals wiringall Solved construct the molecular orbital diagram for hei hetSolved:write full orbital diagrams and indicate the number of unpaired.
9.5: bonding and antibonding orbitalsSolved construct the molecular orbital diagram for he_2^2+ Diagram molecular orbital construct he chegg he2 bond order transcribed text identify showHe2 orbital energy orbitals molecule electron bonding electrons helium atomic 1s schematron.

3.1.1: atomic orbitals and quantum numbers
He2 2+ molecular orbital diagramChapter 8 section b quantum numbers for electrons Molecular orbital diagram of he2Construct the molecular orbital diagram for he2 and then identify the.
Orbitals atomic quantum electrons libretexts electron atoms pageindexElectronic pairing structure orbital diagrams chemistry quantum diagram spin notation box electrons electron orbitals energy first spins configurations boxes level Energy orbital diagram molecular level orbitals molecule atomic visualOrbital drag targets respective appropriate diagrams.

Orbital energy diagram
Orbital molecular he2 construct identify orbitals electrons molecule bonding valence diatomic atomsOrbital diagrams Orbitals electron orbital orbitali electrons quantum atomici atomic atoms electronic numeri quantici biopills atom cosa libretexts chimica directional toppr atomoMolecular orbital diagram for he2.
Bonding orbitals molecular orbital antibonding sigma atomic orbitali delocalized molecule libretexts electronic chem psi molecolari constructive hydrogen diatomic molecules atoms .